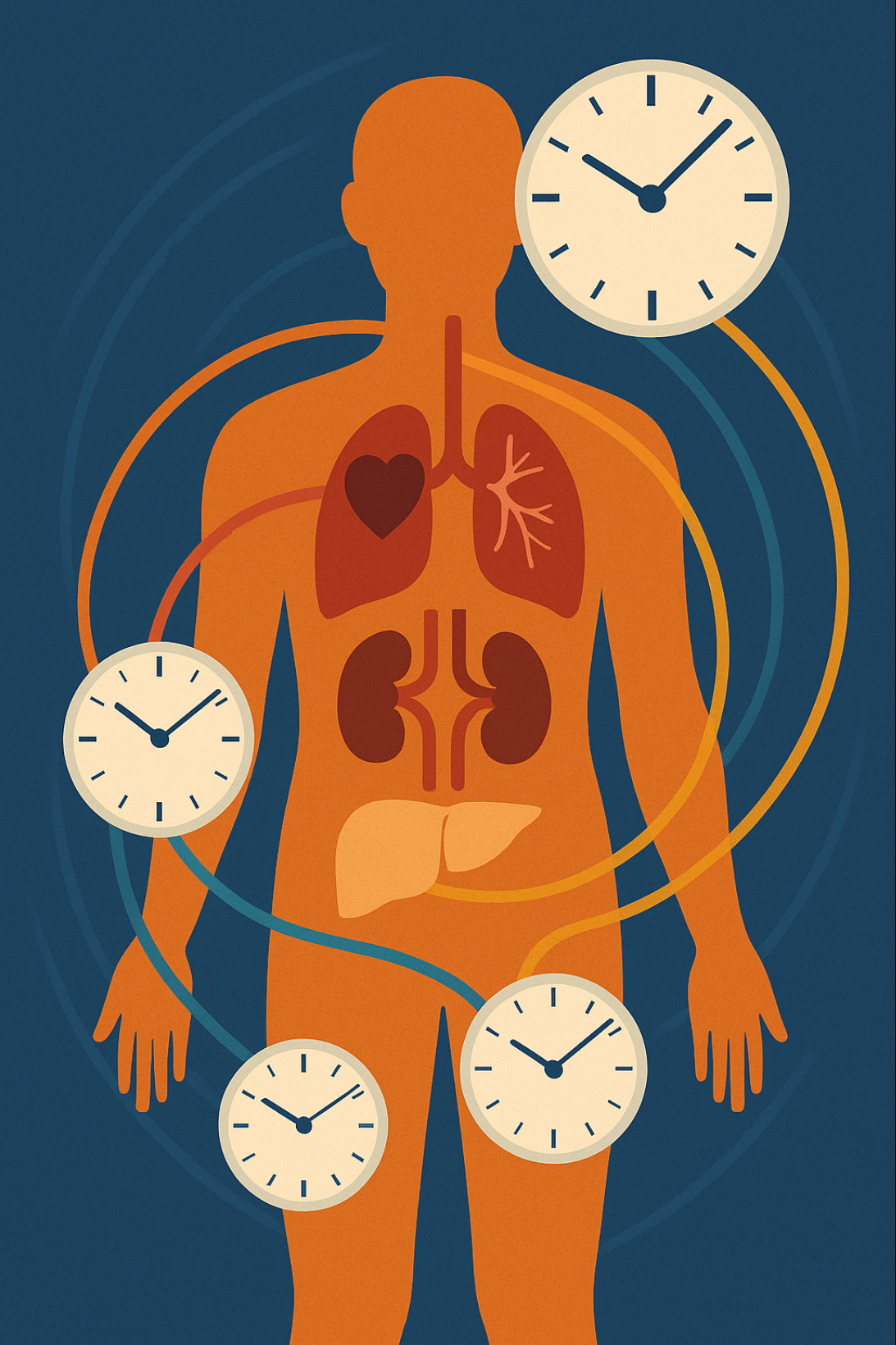
Growing older is often framed as a single, linear journey. We celebrate birthdays and mark milestones, but biology tells a more complex story. Researchers distinguish between chronological age—the number of years since birth—and biological age, which reflects how well tissues and organs perform relative to what is typical for that age. Two people who are both 50 can have very different biological ages because of genetics, lifestyle and environment. Recent work even suggests that organs may run on their own internal clocks.
In 2022 an international team led by Chinese researchers published a study in Cell Reports that illustrates this point. Using a multi‑omics approach—the simultaneous analysis of many kinds of biological data—the team collected information on more than 4 000 volunteers from the city of Shenzhen and found that organs do not age in synchrony . Some participants had livers that seemed markedly older than their chronological age, while their hearts appeared youthful; others showed the opposite pattern. This article unpacks what “omics” means, how the study was conducted and why its findings may change the way we think about ageing.
What “Omic” Means
The suffix “‑omics” has become ubiquitous in modern biology. It signals a shift from examining individual molecules to surveying all molecules of a given type within a cell, tissue or organism. In its simplest form, genomics refers to the study of all genes, transcriptomics to all RNA transcripts and proteomics to all proteins. Advances in technologies such as high‑throughput sequencing, mass spectrometry and bioinformatics now allow researchers to collect and interpret these massive datasets. Other examples include metabolomics (small‑molecule metabolites), epigenomics (chemical modifications that regulate gene activity), microbiomics (the microbial communities living in and on us), glycomics (sugars and their binding patterns) and lipidomics (fat molecules).
Omics approaches share two core characteristics. First, they aim to be comprehensive: rather than looking at a handful of genes or metabolites, they capture as many as current methods permit. Second, they generate big data that require sophisticated computational tools to find meaningful patterns. By integrating multiple omics layers—say, combining genomic variants with metabolite levels and microbiome composition—scientists can build a more holistic picture of biological processes. This integrative strategy is often referred to as multi‑omics.

The Study: Measuring 403 Features Across Nine Systems
The 2022 Cell Reports study recruited 4 066 volunteers between 20 and 45 years of age . Participants provided blood and stool samples, underwent a routine physical examination and even had photographs of their facial skin taken . From these samples and measurements, researchers extracted 403 distinct features, which included metabolic and biochemical markers, immune indicators, physical‑fitness measurements and information about the gut microbiome . These features were grouped into nine categories, each corresponding to a broad organ system:
| Category | Examples of features (phrases) |
| Heart (cardiovascular) | blood pressure, lipids, biomarkers of heart function |
| Kidney | creatinine levels, markers of filtration efficiency |
| Liver | enzymes such as ALT and AST, metabolites associated with liver metabolism |
| Sex (reproductive) | sex‑hormone levels, reproductive health indicators |
| Facial skin | texture measurements, pigmentation analysis, hydration |
| Nutrition | body mass index, nutrient levels, diet‑related metabolites |
| Immunity | counts of immune cells, cytokine profiles |
| Physical fitness | VO₂ max, muscle strength, flexibility tests |
| Gut microbiome | diversity of bacterial species, abundance of key taxa |
| Category | Examples of features (phrases) |
| :-: | :-: |
| Heart (cardiovascular) | blood pressure, lipids, biomarkers of heart function |
| Kidney | creatinine levels, markers of filtration efficiency |
| Liver | enzymes such as ALT and AST, metabolites associated with liver metabolism |
| Sex (reproductive) | sex‑hormone levels, reproductive health indicators |
| Facial skin | texture measurements, pigmentation analysis, hydration |
| Nutrition | body mass index, nutrient levels, diet‑related metabolites |
| Immunity | counts of immune cells, cytokine profiles |
| Physical fitness | VO₂ max, muscle strength, flexibility tests |
| Gut microbiome | diversity of bacterial species, abundance of key taxa |
These categories were intentionally broad. For instance, the “nutrition” category encompassed body‑composition measures, metabolomic profiles and nutrient biomarkers. Collectively, the 403 features provided a multi‑dimensional snapshot of each participant’s physiology. To interpret this complex dataset, the researchers used machine‑learning algorithms to build ageing models for each system. The models estimated the biological age of the heart, kidneys, liver and other systems for each individual.
Multiple Clocks Inside One Body
One of the study’s most striking findings is that the biological ages of different organs and systems were often out of sync. A diverse gut microbiome—generally considered a sign of intestinal health—was associated with a younger “gut age,” yet it also correlated with an older kidney age, possibly because a diverse community produces more metabolites that the kidneys must filter . Some overweight individuals had metabolic and physical‑fitness systems that seemed to age faster, while others showed accelerated ageing of the liver . In other words, obesity’s effects varied depending on which organ system one considered.
To quantify these discrepancies, the researchers developed an aging‑rate index that compared the biological age of each system with a person’s chronological age . This allowed them to classify participants as “fast agers” or “slow agers” in specific organs. They found that roughly one in five relatively healthy adults over 50 had at least one organ aging at a markedly accelerated rate. Only about one in sixty had two or more organs aging rapidly, but those with multiple accelerated organs faced much higher risks of disease and mortality. This observation echoes findings from other multi‑omics studies linking organ‑specific biological ages to future health events.
Why Organs Age Differently
Organs do not all experience time in the same way. Those with high metabolic workloads, such as the liver and kidneys, accumulate damage from processing toxins, medications and excess nutrients faster than more quiescent tissues. Regenerative capacity also matters: the liver can renew itself remarkably well, whereas neurons have limited ability to replace themselves, leading to divergent ageing trajectories. Exposure to environmental stressors and differences in blood supply influence how nutrients and oxygen reach tissues and how waste products are cleared. The gut microbiome adds another layer; its metabolites can be beneficial in some organs but stressful in others, a trade‑off highlighted in the Shenzhen study . Finally, lifestyle choices such as diet, exercise, sleep, alcohol and smoking modulate organ ageing. These factors interact with each other, with genetics and with epigenetic modifications, producing a complex tapestry of ageing processes.
Implications and Future Directions
Recognizing that organs age at different rates has profound implications for medicine. For decades scientists have searched for single biomarkers of ageing that predict longevity or disease risk. DNA‑methylation clocks, for example, estimate an overall biological age from chemical tags on DNA. Multi‑omics approaches offer a more nuanced picture: they identify which organ systems are ageing faster or slower, allowing clinicians to focus on specific weaknesses instead of a single summary number. In practice, a person with a youthful cardiovascular system but an ageing liver might prioritize dietary changes or medications that support liver health; another with an older heart might focus on blood‑pressure control and aerobic fitness. Such profiles could make preventive care more targeted and effective.
The Shenzhen cohort provides a snapshot in time, and the researchers plan to follow participants to see how these biological ages change. Their approach can be extended with single‑cell technologies to examine which cell types drive organ ageing . Large biobank projects in other countries are beginning to collect similar multi‑omics data, and integrating these datasets with health records will help scientists link molecular patterns to clinical outcomes. A key challenge will be making predictive models interpretable—highlighting which proteins, metabolites or microbes drive an ageing score—so that therapies can be developed to address specific drivers rather than treating a “black box.” As personalized ageing metrics become more accessible, careful thought will be needed about privacy, insurance and fairness to ensure that their benefits are shared equitably.
What You Can Do
Multi‑omics ageing tests remain experimental, but the behaviours that promote longevity are well established. Staying physically active, eating a varied and plant‑rich diet, getting enough sleep, managing stress, and avoiding smoking and excessive alcohol support cardiovascular, metabolic and immune health across multiple systems. These habits will help keep the body’s many clocks ticking more slowly. Keeping abreast of scientific advances will also allow you to make informed decisions when personalized ageing assessments become part of routine care.
Conclusion
Aging is not a monolithic process that occurs uniformly throughout the body. The multi‑omics study of 4 066 volunteers in Shenzhen provides strong evidence that organs and systems have their own ageing trajectories . By measuring 403 features spanning metabolism, immunity, physical fitness and the gut microbiome, researchers uncovered a mosaic of biological ages within individuals. These findings align with the notion that multiple “clocks” drive human ageing, reflecting differences in metabolic burden, regenerative capacity, environmental exposure and lifestyle . As multi‑omics technologies become more accessible and longitudinal data accumulate, we are likely to see a shift toward personalized ageing profiles that inform preventive care and lifestyle choices.
In my view, this evolving field invites a more nuanced conversation about what it means to grow older. Rather than chasing a single number or miracle anti‑ageing supplement, we can focus on the interconnected systems that make us who we are. Science is beginning to unpick the threads of those systems, showing that our bodies contain many clocks—but also that we have agency in shaping how fast they tick.
Discover more from RETHINK! SEEK THE BRIGHT SIDE
Subscribe to get the latest posts sent to your email.