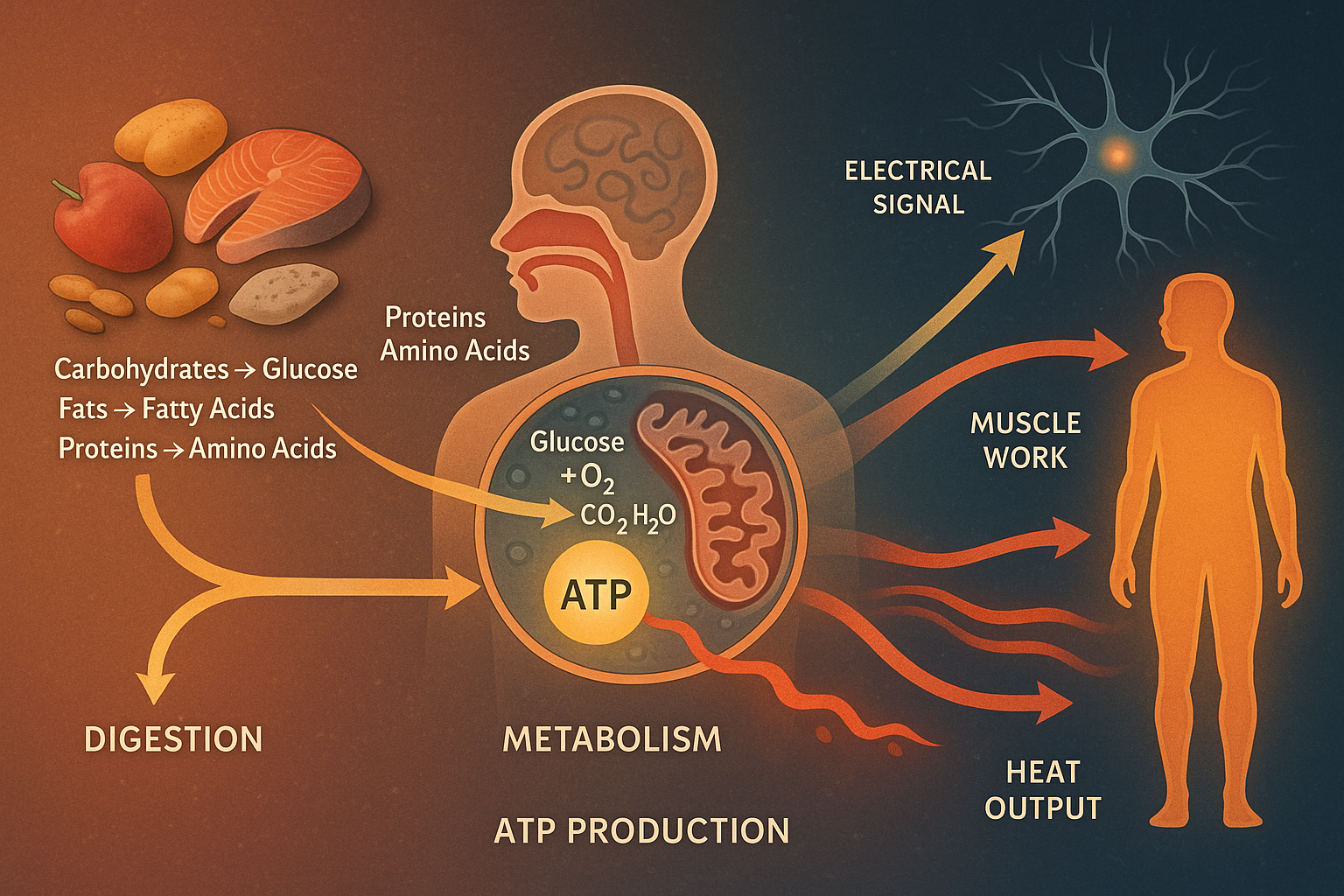
Subtitle: The Journey of Energy in the Human Body—from Digestion to Electrical Impulses, Muscle Motion, and Heat
Living bodies are like energy conversion machines. We take in chemical energy from food and convert it into other forms: kinetic energy of moving muscles, electrical energy in nerves, and thermal energy (body heat) as waste . For example, muscles convert chemical energy to motion, and the eye converts light (optical energy) into electrical nerve signals . Energy also comes in chemical form (in molecules like ATP, carbohydrates, fats) and can be stored briefly in stretched muscles or even as elastic energy. The chart below lists common biological energy forms:
- Chemical energy: stored in molecular bonds (e.g. glucose, fats, and in ATP). Cells free this energy to do work .
- Mechanical energy: visible as movement (muscles doing work, the body walking, blood flowing). Muscles literally convert ATP into mechanical work .
- Electrical energy: used in nerves and muscle cells as voltage across membranes. For instance, neurons use ATP to set up ion gradients that store electrical potential (like a battery) .
- Thermal energy (heat): generated by metabolism. Most chemical processes are inefficient, so some energy “spills out” as heat. This keeps us warm and is a byproduct of most ATP use .
- Other forms: Light absorbed by the eye or sound waves caught by the ear are converted into neural (electrical) signals . In short, energy in biology is fundamentally chemical at the start, then shuttled into motion, electricity, or heat as needed.
Cellular Energy Production: Respiration
At the cellular level, energy comes from cellular respiration, the controlled breakdown of nutrients to make ATP. In humans (and all animals), glucose is a primary fuel. Cells “burn” glucose with oxygen to make carbon dioxide, water, and energy . Unlike a fireball, this release is done in many small steps inside the cell. Each step (glycolysis, the Krebs cycle, and the electron transport chain) strips off electrons and uses them to make ATP . The overall reaction is roughly:
Glucose + O₂ → CO₂ + H₂O + (energy in ATP and heat).
Most of the energy released ends up stored in new ATP molecules . Aerobic respiration (with oxygen) is very efficient – up to ~30–32 ATP can be produced from one glucose . By contrast, without oxygen (in fermentation), only 2 ATP per glucose are made, and much energy remains locked in byproducts like lactate . In sum, cells convert food chemicals into the chemical energy of ATP rather than blasting it out as light or fire.
Below is a schematic of the main stages of respiration:
- Glycolysis (in cytoplasm): Glucose (6-carbons) is split into two pyruvates (3-carbons), generating 2 ATP and high-energy electrons (carried by NADH).
- Krebs (Citric Acid) Cycle (in mitochondria): Pyruvate is fully oxidized to CO₂, producing 2 more ATP (directly) plus many electron carriers (NADH, FADH₂).
- Electron Transport Chain (in mitochondrial inner membrane): Electrons from NADH/FADH₂ flow down a chain to oxygen, releasing energy used to make ~26–28 more ATP. Oxygen finally accepts the electrons and protons to form water.
All living cells – animals, plants, and microbes – run these processes (or analogs) to power life . In plants, the initial “fuel” may be made by photosynthesis; in animals, it comes from our diet.
ATP: The Cellular Energy Currency
The ATP molecule (adenosine triphosphate) is the key energy carrier. Structurally, ATP has an adenosine group linked to three phosphate groups. The bonds between these phosphates are high in chemical potential. When ATP is hydrolyzed (broken by adding water) to ADP (adenosine diphosphate) and a free phosphate, energy is released that can drive other reactions. You can picture ATP as a rechargeable battery or an “energy coin” that the cell spends. Just as a rechargeable battery stores electrical energy, ATP stores chemical energy from food; when needed, the cell “cashes” an ATP and uses the energy.
Figure: Structure of ATP (adenosine triphosphate) showing the adenine base, ribose sugar, and three phosphate groups (often labeled α, β, γ). Breaking the bond between the last two phosphates releases energy, making ATP the cell’s energy currency .
ATP captures chemical energy from food breakdown and then delivers it wherever needed inside cells . Notably, ATP itself is not a long-term storage form (that role belongs to carbohydrates and fats) . Instead, when a cell needs energy, it quickly converts stored fuels into ATP. ATP then shuttles this energy to power processes like synthesizing molecules, pumping ions, or moving fibers .
ATP Hydrolysis: Energy Release
The hydrolysis of ATP (ATP + H₂O → ADP + P_i + energy) is an exergonic reaction: it releases free energy because the products (ADP and inorganic phosphate) are more stable (lower energy) than ATP . Under typical cellular conditions, each ATP hydrolysis liberates on the order of 30–60 kJ/mol of free energy . In practical terms, breaking one ATP “saves” enough energy to do useful work in the cell. For example, muscle myosin uses each ATP to change conformation and pull on actin filaments .
Approximately 60% of the energy from ATP hydrolysis dissipates as heat, even in healthy cells . In other words, only a fraction (often around 40%) is harnessed for work; the rest warms the cell or body. This heat is part of why warm-blooded animals must eat constantly and why we feel warmer when muscles work hard. Importantly, ATP hydrolysis itself produces no light or sound – the energy comes out chemically and thermally, and can be immediately used or lost as heat.
Because cells pair reactions, ATP hydrolysis often drives endergonic (energy-requiring) processes via coupling. For instance, ATP’s terminal phosphate can be transferred (phosphorylated) onto a protein or small molecule. The recipient then undergoes a change that wouldn’t occur spontaneously. A classic example is the sodium-potassium pump: this membrane protein uses the energy from ATP hydrolysis to transport Na⁺ and K⁺ ions against their gradients .
Using Chemical Energy: Cellular Work
Once ATP’s phosphate bonds are broken, the released chemical energy is funneled into three broad categories of cellular “work” :
- Biosynthesis (chemical work): Cells use ATP to build macromolecules (proteins, nucleic acids, lipids, etc.). Joining amino acids into a protein chain, for example, requires ATP to activate substrates and form new bonds. In general, any endergonic metabolic reaction (one that wouldn’t occur on its own) is driven by coupling to ATP or related carriers .
- Active transport (transport work): ATP fuels pumps that move substances across membranes. The Na⁺/K⁺ ATPase (ubiquitous in animal cells) is driven by ATP: hydrolysis transfers a phosphate to the pump, causing it to expel 3 Na⁺ out and bring 2 K⁺ in . Without ATP, cells could not maintain the ionic gradients that underlie nerve impulses and cell homeostasis. Other examples include Ca²⁺-ATPases that clear calcium from muscle cells and proton pumps in bacteria or cell membranes.
Figure: The sodium-potassium ATPase in the cell membrane. In this illustration the pump (blue protein) uses the free energy from ATP hydrolysis to move Na⁺ (green) out and K⁺ (purple) in, against their gradients . This is a form of active transport powered by ATP.
- Mechanical work: Many cellular movements use ATP. In muscle cells, myosin motor proteins bind and hydrolyze ATP to “cock” and pull actin filaments , causing contraction. Essentially, each ATP molecule gives the myosin head enough energy to perform a power stroke (the force-producing step). Similarly, motor proteins like kinesin and dynein use ATP to walk along microtubules, transporting vesicles within cells. Even cilia and flagella beat thanks to ATP-driven motors.
In practice, processes often combine categories. For example, the beating of a heart muscle cell uses ATP (mechanical work) but also relies on pumping Ca²⁺ (active transport) to reset each heartbeat. Building new molecules in a nerve cell supports its signaling (biosynthesis + electrical function).
In short, cells convert the chemical energy of ATP into the specific energy needed: making things (biosynthesis), moving things (transport or mechanical motion), or creating signals. Any leftover energy becomes heat.
Energy Use in Major Organs
Different organs emphasize different energy uses:
- Brain: Although the brain is only ~2% of body weight, it uses about 20% of the body’s energy . Almost all of this energy goes to electrical signaling. Neurons continuously pump Na⁺ and K⁺ ions to maintain membrane potentials (essential for nerve impulses). ATP provides the energy for these ion pumps . In fact, research shows roughly two-thirds of a neuron’s ATP fuels signal propagation, while the rest goes to cellular “housekeeping” (making neurotransmitters, membrane maintenance, etc.) . The brain relies almost entirely on glucose (and ketones during starvation) and must run aerobic respiration (it cannot ferment efficiently).
- Heart: The heart muscle (myocardium) is an endurance champion. Under normal oxygen, >95% of the heart’s ATP comes from oxidative phosphorylation in mitochondria . The heart consumes a huge amount of oxygen and prefers fatty acids as fuel (they yield more ATP per molecule). About 60–70% of cardiac ATP is used for contraction (pumping blood) and the rest for ion pumps and other work . The heart constantly runs, so it has an enormous density of mitochondria (the “power plants” of the cell).
- Skeletal Muscle: Muscle cells store some energy in creatine phosphate for immediate use, but mostly they, too, rely on respiration. At rest or during moderate exercise, muscles use aerobic metabolism (burning glucose and fats with oxygen). During intense short bursts (sprinting or heavy lifting), muscles can’t get oxygen fast enough and switch partly to anaerobic metabolism (glycolysis). This leads to lactic acid production and only 2 ATP per glucose . That less-efficient mode provides quick bursts of power but also generates heat and acidity (the “burn”). In summary, skeletal muscle is versatile: it can sustain prolonged work using oxygen or quickly power movements anaerobically.
Other organs have their energy quirks. For instance, the liver both stores and releases energy (converting between glucose and glycogen) and does lots of biosynthesis. Adipose (fat) tissue stores energy in lipids and even consumes glucose to maintain fat stores.
Other Forms of Biological Energy
Beyond chemical energy from ATP, human physiology involves other energy forms, all rooted in ATP’s power:
- Electrical energy in nerves: Neurons use ATP to set up ion gradients, creating voltage differences across membranes. When a nerve fires, this chemical energy is converted to an electrical signal that travels along the axon. In effect, neurons “charge up” like tiny batteries. (Photoreceptor cells in the eye convert light into electrical signals , and hair cells in the ear convert sound waves to neural signals .)
- Heat production (thermoregulation): Because metabolism is not 100% efficient, much of our energy intake ends up as heat. We use this to maintain a constant body temperature. For example, when you shiver in the cold, rapid muscle contractions burn ATP and produce heat. Humans also have brown fat (especially infants do), which burns fuel to generate heat without muscular work. In all cases, the source of heat is the chemical energy of ATP being dissipated.
- Kinetic motion in the bloodstream: Although not often singled out, blood flow and breathing also embody mechanical energy. The heart’s pumping motion (mechanical energy) sends blood surging through vessels. These motions ultimately come from ATP-driven muscle contractions.
No form of energy in our bodies appears magically; it all originates from chemical energy in nutrients (and ultimately ATP) and is transformed via the processes above.
Evolutionary and Comparative Perspectives
The ATP-based energy scheme is universal in life, but different organisms have adapted it in various ways:
- Anaerobic vs Aerobic: Many microbes and lower animals rely on anaerobic pathways. For instance, yeast ferments sugar to ethanol and CO₂, yielding just 2 ATP per glucose . This is far less efficient than human respiration (~30 ATP), but it works without oxygen. Some animals, like hard-working muscles or swimming fish, can temporarily tolerate high lactate until oxygen returns. Humans, by contrast, evolved to use oxygen constantly, making much more ATP per meal and sustaining high metabolism.
- Photosynthesis vs Respiration: Plants capture sunlight to produce ATP (via the chloroplast’s light reactions) and build sugars. They then break down those sugars via cellular respiration just as animals do. This contrast (sunlight → ATP → sugar vs. eating food → ATP) highlights how life has two strategies to make chemical energy.
- Endotherms vs Ectotherms: Mammals and birds keep a steady high body temperature by burning fuel (metabolic heat). This demands more food but allows biochemical processes to run at optimal rates. Reptiles and amphibians often rely on external heat (sunlight) and have slower metabolisms. For example, a lizard sitting in the sun charges its “ATP battery” more slowly, and it may bask to raise its body temperature for better muscle performance. Hibernating mammals cut way back on metabolism (and ATP use) in winter to save energy. These adaptations show that how much and how ATP is used can vary greatly by evolutionary niche.
- Specialized fuels: Even among animals, fuel preference varies. As noted, the heart loves fats, while the brain relies on glucose (with ketones in famine). Marine mammals have blubber for energy stores, and migratory birds rapidly build fat to fuel long flights (each fat molecule yields far more ATP than one glucose).
In all cases, however, the core principle is the same: chemical energy in nutrients (or sunlight) is converted into ATP, which then powers life’s diverse processes. The variations (e.g. lots of mitochondria, alternative fuels, different enzymes) are refinements shaped by evolution to meet each organism’s needs.
Sources: Scientific textbooks and articles on bioenergetics and physiology. Key points are supported by texts such as LibreTexts and Britannica , which document ATP’s role as the energy carrier, the ATP hydrolysis process, and how different cells and organs use that energy. These resources ensure the details above are accurate and up-to-date.
Discover more from RETHINK! SEEK THE BRIGHT SIDE
Subscribe to get the latest posts sent to your email.