Theranostic Porphyrin–Lipid Nanoparticles (Por-NPs) for Atherosclerosis
Introduction
A new generation of “theranostic” nanoparticles has been shown to both detect and reduce plaques in the arteries . These porphyrin-lipid nanoparticles (Por-NPs) are designed to target atherosclerotic plaques and serve a dual purpose: imaging diseased sites and delivering therapy simultaneously. Atherosclerosis, the buildup of fatty inflamed plaques in artery walls, remains a leading cause of heart attacks and strokes . Traditional treatments like statins lower cholesterol but cannot directly visualize or eliminate existing high-risk plaques once they form . Por-NPs aim to fill this gap by identifying dangerous plaques and intervening to shrink them, breaking the vicious cycle of inflammation and plaque growth .
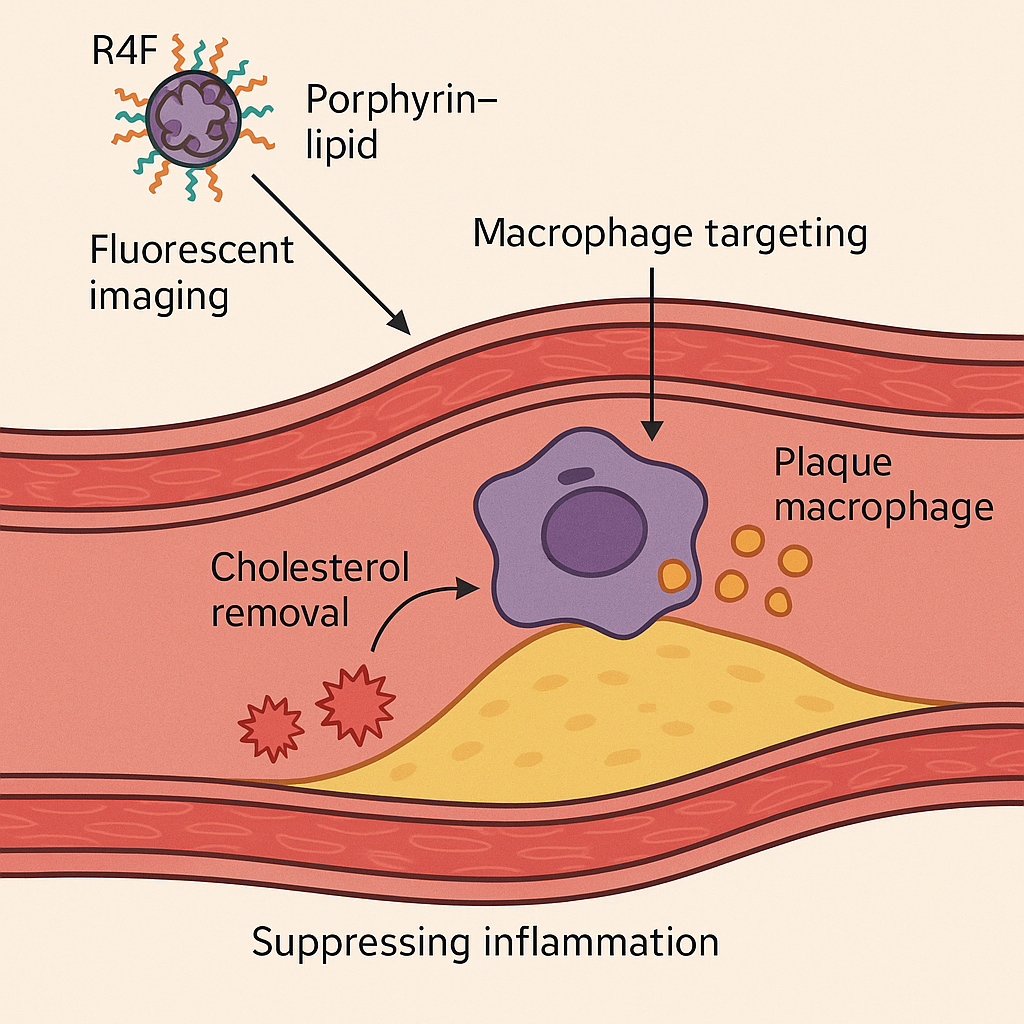
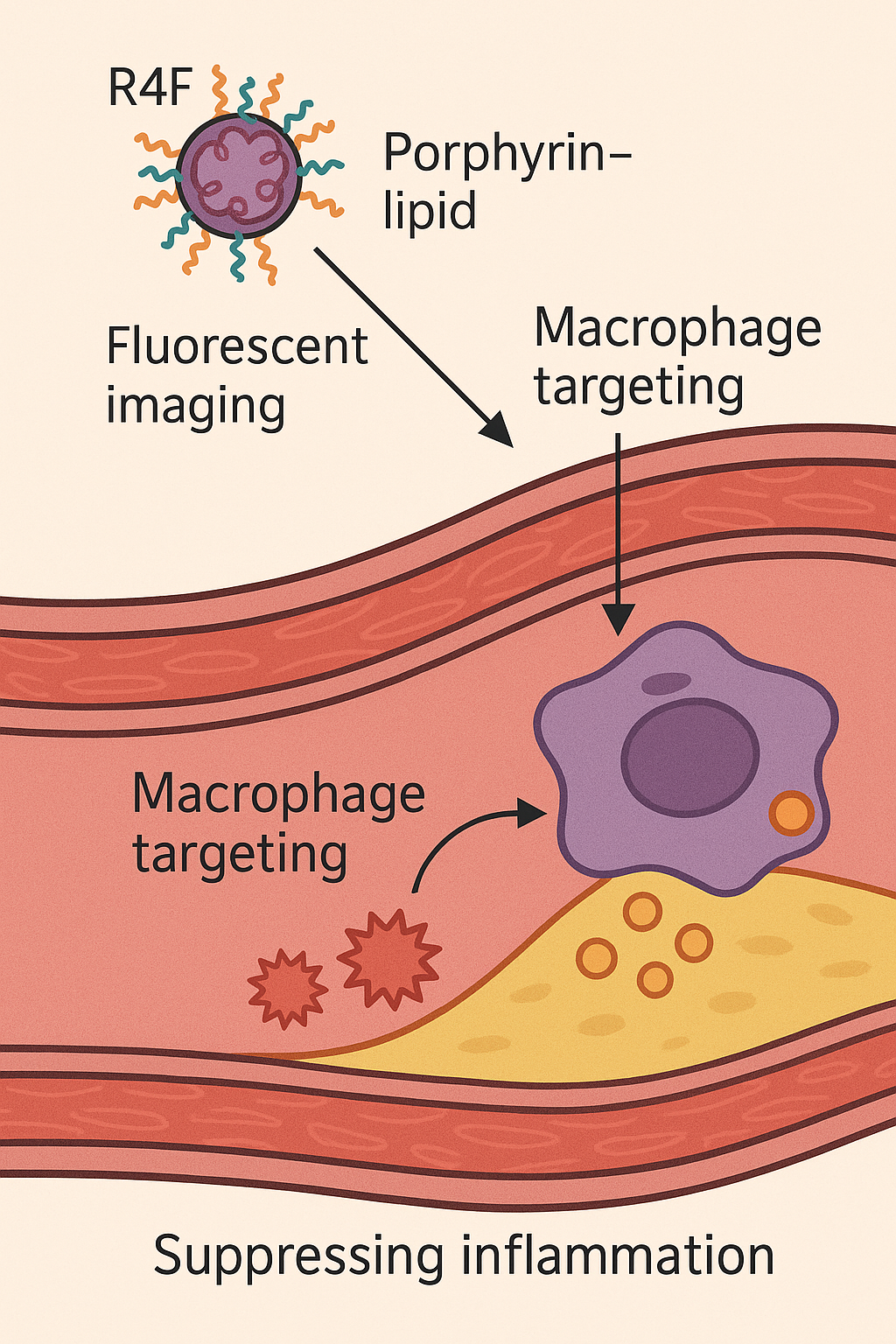
Illustration of how Porphyrin–lipid nanoparticles (Por-NPs) treat atherosclerotic cardiovascular disease by targeting plaque macrophages, promoting cholesterol removal, and suppressing inflammation (adapted from Nankivell et al.,
Materials Today Bio ). This theranostic nanoparticle platform combines a fluorescent, radiometal-chelating porphyrin-lipid core with an apolipoprotein-mimetic peptide shell. The unique design enables multimodal imaging of plaques (optical fluorescence and PET scanning) alongside biological therapy at the disease site.
Nanoparticle Design and Targeting Mechanism
Porphyrin-lipid nanoparticles are built to mimic key functions of high-density lipoprotein (HDL) and specifically seek out plaque macrophages. The nanoparticle’s core contains a porphyrin–lipid conjugate that naturally emits near-infrared fluorescence and can chelate Copper-64 for PET imaging . This allows the particles to be tracked in living tissue via both fluorescent imaging and positron emission tomography . The outer shell is decorated with a short peptide called R4F, an apolipoprotein A-I mimetic (also known as “reverse 4F”) that is designed to bind the scavenger receptor SR-BI on macrophages . By targeting SR-BI – a receptor abundantly expressed on plaque macrophages – the R4F coating directs the nanoparticles to atherosclerotic lesions for selective uptake . These Por-NPs are extremely small (~20 nm in diameter) , which is much smaller than a typical virus, facilitating deep penetration into plaques.
Once a Por-NP is taken up by a plaque macrophage, it effectively functions like a miniature HDL particle inside the cell. The R4F/SR-BI interaction and HDL-mimetic design help the foam cell offload excess cholesterol and lipids, preventing these macrophages from becoming overloaded “foam cells” . At the same time, the nanoparticle’s presence modulates the cell’s inflammatory pathways, helping to suppress the inflammatory response that drives plaque progression . Notably, researchers found that the anti-inflammatory effects of Por-NPs are partly independent of SR-BI and cholesterol efflux pathways, suggesting these particles may also neutralize pro-inflammatory signals directly .
Diagnostic Imaging Capabilities
Porphyrin-lipid NPs are intrinsically traceable by imaging, enabling early diagnosis of atherosclerosis and monitoring of treatment response. The porphyrin-based core fluoresces under infrared light and can be radiolabeled with ^64Cu, giving the particles a built-in dual imaging modality . In preclinical studies, infused Por-NPs were clearly visualized within plaques. PET scans detected the accumulation of ^64Cu-labeled Por-NPs in the hearts and arteries of atherosclerotic mice, and could even track the progression of plaque burden over time . Similarly, near-infrared fluorescence imaging revealed strong signals from Por-NPs co-localizing with CD68^+ macrophages inside plaque regions . This demonstrates that Por-NPs can serve as a contrast agent marking inflamed, macrophage-rich plaques using multiple imaging techniques. Importantly, the nanoparticles’ multimodal imaging capability could allow cardiologists to identify high-risk plaques earlier and more specifically than traditional scans .
Therapeutic Effects in Atherosclerosis Models
Beyond imaging, Por-NPs deliver tangible therapeutic benefits by altering plaque biology. In vitro experiments with macrophages showed that Por-NPs are readily internalized by these immune cells . Once inside, the particles significantly enhanced cholesterol efflux from the macrophages – by about 49% more than even natural reconstituted HDL particles . This means foam cells were able to unload a greater share of their cholesterol, which is a key step in stabilizing or regressing plaques.
Crucially, Por-NPs also damped inflammatory signaling in macrophages. Treated macrophages had dramatically lower levels of inflammatory cytokines: for example, mRNA for IL-1β was reduced by 88% and CCL5 (RANTES) by 75%, with corresponding protein levels of these cytokines dropping ~70–80% relative to controls . The nanoparticles suppressed activation of the NF-κB pathway and inflammasome components (e.g. NLRP3 by ~69%), which are central drivers of inflammation in atherosclerosis . Interestingly, follow-up tests revealed that these anti-inflammatory effects did not require SR-BI or cholesterol removal to occur . In other words, the Por-NPs inherently carry anti-inflammatory properties—likely by sequestering toxic lipid byproducts or directly interfering with inflammatory signaling—beyond just their HDL-like cholesterol transport function.
When tested in living animals, the Por-NPs demonstrated striking atheroprotective effects. Atherosclerosis-prone ApoE^-/- mice on a high-fat diet were treated with these nanoparticles in two disease models: one simulating early-stage plaque development and another modeling advanced, unstable plaques . In the early-stage disease group, mice receiving Por-NPs developed significantly smaller plaques (approximately 22% smaller by area) compared to untreated mice . In the advanced plaque model – which mimics vulnerable plaques prone to rupture – treated mice showed a remarkable 52% reduction in plaque size versus controls . These therapeutic effects on plaque burden were accompanied by a systemic anti-inflammatory impact: treated mice had 32% fewer circulating monocytes (inflammatory white blood cells) and an 81% reduction in monocyte/macrophage content within plaques relative to controls . Genes that drive inflammation in arterial walls were also “dialed down” in treated animals, indicating a healthier plaque environment .
Notably, the fate of the cholesterol removed from plaques appears to be benign. After doing their work in the arteries, the Por-NPs (and the cholesterol they carry) were mostly transported to the liver for disposal, just as natural HDL would do . Researchers observed no harmful accumulation in the liver or other organs, suggesting the nanoparticles safely integrate into the body’s normal cholesterol metabolism and clearance routes . As Dr. Victoria Nankivell, the study’s lead author, summarized: “These nanoparticles don’t just detect arterial plaque… they can also suck it up and take it to the liver, lowering inflammation” .
Future Directions and Clinical Translation
The promising results with Por-NPs so far have been achieved in preclinical (in vivo) models of atherosclerosis – primarily mouse models of arterial plaque disease . The ability to simultaneously image and treat plaques marks an exciting step toward so-called “theranostic” cardiology. According to the investigators, Porphyrin-lipid NPs represent a potential nanoscale theranostic platform for atherosclerotic cardiovascular disease . In other words, a single agent can play both diagnostic and therapeutic roles, as demonstrated by its multimodal plaque imaging and atheroprotective effects in animals .
Having shown efficacy in animals, the next challenge is to translate this nanoparticle technology into clinical trials. The research team is now exploring further development of Por-NPs for clinical use, with the goal of complementing existing heart disease treatments and improving patient outcomes . Many steps remain, including scaling up nanoparticle production, rigorous safety evaluation, and regulatory approval before human studies can begin. Nonetheless, the concept holds great potential. In the future, Por-NPs (or similar HDL-mimetic nanomedicines) could be used alongside standard therapies to not only visualize dangerous plaques non-invasively but also actively reduce plaque size and inflammation before they cause heart attacks or strokes . This approach exemplifies the growing field of nanomedicine, where tiny engineered particles are employed to both see and treat disease processes at the same time .
Key Points
- Diagnostic capability: Yes. Porphyrin-lipid NPs can be tracked by near-infrared fluorescence and PET imaging, enabling identification of atherosclerotic plaques in vivo .
- Therapeutic capability: Yes. The R4F-coated NPs actively reduce plaque burden and inflammation by targeting cholesterol-laden macrophages and modulating their biology .
- Dual function (Theranostic): Both. These nanoparticles serve as a combined diagnostic and therapeutic tool – one platform for imaging plaques and treating them simultaneously .
- Stage of development: Tested in vivo in animal models (mice), showing significant efficacy . Now under development towards clinical trials, with hopes to translate this approach into human heart disease management.