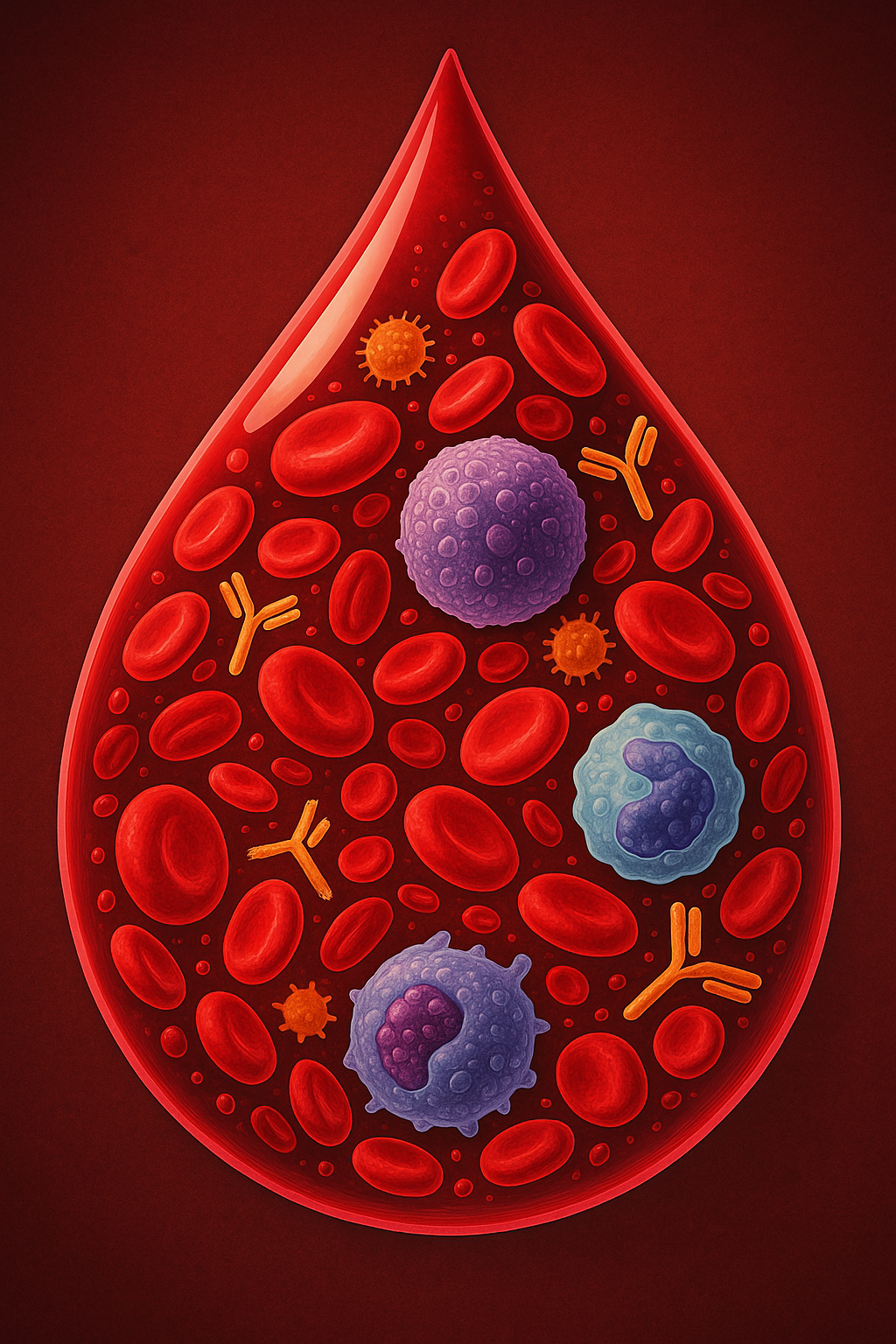
At first glance, a drop of blood looks like a tiny red bead, but under the microscope it is teeming with life. Imagine swirling through that crimson drop and seeing about 400 000 immune cells moving with purpose among 250 million red blood cells and 15 million platelets. Even more astonishing, each drop contains roughly 13 trillion antibodies. These immune components patrol our bloodstream day and night, constantly scanning for invaders and coordinating defenses. This imaginary journey highlights how deeply our bodies invest in immunity—an investment that keeps us alive every second. To appreciate this invisible army, we need to understand who these cells are, what they do and how our daily habits shape them. This article offers a detailed but accessible tour of the immune system for everyone, beginning with its two great divisions and then exploring the specialisation and collaboration that make human immunity so effective.
Two Arms of Defense: Innate and Adaptive Immunity
The immune system has two main divisions. Innate immunity is our first line of defense, acting quickly and in the same way against all germs . Skin and mucous membranes form physical barriers, while acid, enzymes, mucus, cilia, tears and sweat create chemical and mechanical defenses that stop microbes from growing or entering our bodies . When invaders breach these barriers, innate cells respond within hours.
Adaptive immunity, by contrast, is slower but highly specific. After encountering a particular germ, it creates a tailored response and remembers that pathogen so it can respond rapidly upon re‑exposure . Adaptive immunity relies on lymphocytes—T cells and B cells—that recognise unique antigens and work together to neutralise them . Memory cells formed during adaptive responses ensure long‑term protection.
Below we explore the major cell types and molecules in these two systems, highlighting how they cooperate to protect us.
The Foot Soldiers: Innate Immune Cells
Macrophages and Monocytes
Monocytes are large white blood cells produced in the bone marrow. They circulate in the bloodstream for only a day or two before migrating into tissues such as the spleen, where many differentiate into macrophages . Monocytes function as reserve forces—they destroy invaders, remove infected cells and support healing . Some monocytes become dendritic cells, specialising in presenting antigens to T cells .
Once in tissues, macrophages act like Pac‑Man. They surround and digest microorganisms, clear away debris and dead cells, and stimulate other immune and non‑immune cells . Recent research shows macrophages are diverse; different subsets recruit distinct immune cells to particular infections or injuries . Macrophages also “report” what they have eaten by presenting pieces of digested pathogens on their surface, helping to initiate adaptive responses.
Neutrophils: Rapid Responders
Neutrophils are the most abundant white blood cells and are first on the scene when infection strikes . They are classic phagocytes, engulfing bacteria and fungi. To kill invaders, neutrophils produce reactive oxygen species and release antimicrobial proteins, and they can form extracellular traps (NETs)—web‑like structures that immobilise microbes . Because they are short‑lived, dying after a few hours of battle, your bone marrow churns out billions every day.
Eosinophils, Basophils and Mast Cells
Not all invaders are tiny bacteria. Some, like worms and parasites, are much larger. Eosinophils are specialised phagocytes that target these larger parasites with toxic proteins and inflammatory molecules; however, they are also involved in allergy and asthma, where they can damage tissues .
Basophils circulate in the blood and make up less than 1 % of white blood cells. Differentiation from myeloid progenitors is driven by the cytokine IL‑3 . Basophils are activated when IgE antibodies bound to their surface are cross‑linked by allergens; they can also be triggered by complement proteins (C5a and C3a). Once activated, basophils release histamine and produce cytokines such as IL‑4 and IL‑13 . These secretions recruit other immune cells and support responses against parasites but also mediate allergic reactions.
Mast cells are long‑lived immune cells that reside in tissues, particularly at the body’s boundaries (skin, gut, lungs). They develop from bone marrow progenitors but mature in tissues where they rely on stem cell factor (SCF) to survive . When activated by allergens, pathogens or physical stimuli, mast cells release a wide array of inflammatory mediators, including histamine, proteases and cytokines. These mediators defend against parasites but also cause immediate hypersensitivity reactions (allergies). Because mast cells are stationed at entry points, they act as early sentinels, bridging innate and adaptive immunity.
Dendritic Cells: The Professional Antigen Presenters
Dendritic cells (DCs) are among the most potent antigen‑presenting cells in the body. They originate from bone marrow and come in several varieties (myeloid and plasmacytoid) . Immature DCs patrol tissues using endocytosis, macropinocytosis and phagocytosis to sample their environment . When they encounter a pathogen, they migrate to the nearest lymph node, mature and stop capturing antigens. Mature DCs upregulate co‑stimulatory molecules (CD80, CD86) and chemokine receptors such as CCR7, secrete pro‑inflammatory cytokines, and display processed antigens on major histocompatibility complex (MHC) molecules . A single DC can stimulate hundreds to thousands of T cells, acting as a crucial bridge between the innate and adaptive systems .
Natural Killer Cells: Innate Lymphocytes
Natural killer (NK) cells arise from the same progenitor as T and B cells but belong to the innate immune system. They patrol the body and respond quickly to infection or cancer. NK cells recognise stressed or abnormal cells lacking normal MHC I molecules, balancing signals from activating and inhibitory receptors . When activated, NK cells release perforin and granzymes, which create pores in target-cell membranes and trigger apoptosis. They also secrete cytokines like interferon‑γ (IFN‑γ) and tumor necrosis factor‑α (TNF‑α), stimulating macrophages and dendritic cells to improve immune responses .
The Specialist Team: Adaptive Immunity
B Cells and Antibodies
When B cells encounter their specific antigen, often with help from T helper cells, they proliferate and differentiate into plasma cells that secrete large quantities of antibodies. Each antibody binds uniquely to its antigen, neutralising pathogens or marking them for destruction. Some B cells become memory B cells that persist after the infection and provide rapid protection if the pathogen returns .
Antibodies are proteins found in many body fluids including saliva, tears and breast milk . They come in five main classes:
- Immunoglobulin A (IgA) is present in mucosal secretions (saliva, tears, mucus) and breast milk. It protects surfaces of the respiratory, gastrointestinal and reproductive tracts .
- IgD is found on the surface of naïve B cells and appears to support B‑cell activation and maturation .
- IgE is mainly located in the skin, lungs and mucous membranes; it binds to mast cells and basophils and triggers release of histamine, mediating allergic reactions .
- IgG is the most abundant class, circulating in blood and tissues. It crosses the placenta to protect newborns and plays a major role in neutralising viruses and bacteria .
- IgM is produced early in infections; it circulates in blood and lymph and is vital for initial immune defence and regulation .
T Cells: Coordinators, Killers and Regulators
T cells are central to adaptive immunity and exist in different functional types. Helper T cells (CD4⁺) recognise antigen fragments presented by dendritic cells on MHC II. They secrete cytokines that activate B cells, cytotoxic T cells and macrophages . Cytotoxic (killer) T cells (CD8⁺) recognise infected or cancerous cells presenting antigen on MHC I and induce apoptosis . After the response, memory T cells remain to provide faster defence upon re‑infection .
A third subset, regulatory T cells (Tregs), prevents over‑reactions and autoimmunity. Natural Tregs develop in the thymus and express CD4, CD25 and the transcription factor FOXP3. They suppress activation, proliferation and cytokine production of other T cells and may dampen B‑cell and dendritic‑cell activity . Tregs produce inhibitory cytokines like transforming growth factor‑β (TGF‑β), interleukin‑10 (IL‑10) and adenosine and express molecules such as CTLA‑4 and GITR . This regulatory network helps maintain tolerance to self‑antigens and prevents chronic inflammation.
Complement and Cytokines: Chemical Messengers and Clean‑Up Crew
Beyond cells and antibodies, the immune system relies on chemical cascades. Complement proteins are a group of plasma proteins that activate one another to form complexes that punch holes in bacterial membranes. They also mark germs for destruction and attract immune cells to infection sites . Cytokines—tiny proteins released by many immune cells—act as messengers, orchestrating immune responses. For example, interferons inhibit viral replication; interleukins promote communication among leukocytes; and chemokines guide cell migration.
Life Lessons from Immunity: Lifestyle’s Role in Defenses
The immune system does not operate in isolation; it responds to cues from sleep, exercise, stress and nutrition. Here we summarise what science tells us about these influences.
Sleep: The Night Shift for Immunity
Sleep and immunity have a bidirectional relationship. Enough high‑quality sleep strengthens both innate and adaptive immune responses; inadequate sleep weakens them . During sleep, the production of cytokines increases; this inflammatory response aids recovery and reinforces immune memory . Sleep also frees energy by slowing breathing and muscle activity, allowing the immune system to work more efficiently. Conversely, chronic sleep deprivation increases susceptibility to infections; some evidence suggests that poor sleep can reduce vaccine effectiveness. . Prioritising seven to eight hours of sleep each night, establishing regular bedtime routines and creating a dark, cool sleeping environment can bolster immune health.
Exercise: A Force Multiplier
Physical activity influences immunity via inflammation and hormone regulation. Moderate exercise—like brisk walking, cycling or swimming—elicits mild inflammatory responses that strengthen the immune system . In contrast, extended high‑intensity workouts (more than 90 minutes) may temporarily suppress immunity . Studies show that people who engage in light to moderate exercise around vaccination have higher antibody responses and improved vaccine efficacy . Health authorities recommend 150 minutes of moderate activity or 75 minutes of vigorous exercise per week, along with strength training; even walking 6 000–8 000 steps a day benefits immunity . To support your immune system, aim for consistent moderate movement rather than occasional intense sessions.
Stress: The Double‑Edged Sword
Stress triggers the release of cortisol, a hormone that limits inflammation and helps us respond to immediate threats. In short spurts, cortisol can enhance immune readiness; however, chronic stress keeps cortisol elevated, leading to immune dysregulation. Prolonged high cortisol levels reduce lymphocyte (white blood cell) numbers, making you more susceptible to infections such as the common cold . Chronic stress also promotes inflammation and is associated with conditions like arthritis, lupus and fibromyalgia . To shield your immune system, adopt stress management techniques such as meditation, mindfulness, yoga, regular physical activity and a balanced diet . Even a few minutes of deep breathing or walking in nature can reduce cortisol and help immune cells function optimally.
Nutrition: Fuel for the Army
A diverse, balanced diet provides the vitamins, minerals, proteins, fats and carbohydrates that immune cells need. Although this article focuses on biology, it’s worth noting that certain nutrients—such as vitamins A, C and D, zinc, selenium and omega‑3 fatty acids—support immune function. Meanwhile, high consumption of refined sugar and saturated fat can promote inflammation. Eating plenty of fruits, vegetables, whole grains, legumes, nuts and seeds, and staying well hydrated, ensures your immune system has the resources to mount responses and recover.
A Holistic Picture
Your immune system is an astonishing network of cells, proteins and chemical messengers that work together to protect you. It begins with skin and mucous membranes that create physical barriers. If pathogens penetrate these barriers, innate cells like macrophages, neutrophils, eosinophils, basophils, mast cells, dendritic cells and NK cells act quickly. They engulf invaders, release toxic molecules and call for reinforcements. Dendritic cells carry information about pathogens to lymph nodes, where they activate the adaptive arm. B cells and T cells collaborate to mount targeted responses; antibodies neutralise pathogens or mark them for destruction, while cytotoxic T cells kill infected or cancerous cells. Regulatory T cells keep the system in balance to prevent autoimmunity. Complement proteins and cytokines act as chemical amplifiers and messengers.
This complex machinery can be strengthened or weakened by our daily choices. Sufficient sleep, consistent moderate exercise and stress management help immune cells perform optimally. A balanced diet fuels their activity, while chronic stress or sleep deprivation can impair defenses. Taking care of your immune system isn’t just about avoiding germs—it’s about creating a lifestyle that supports the incredible universe of cells and molecules working tirelessly in each drop of your blood.