Recent developments in dental technology have led to the creation of bioengineered teeth that can “grow” in place,
—A comprehensive review of the science, milestones, and future clinical pathway of living tooth regeneration
1. An unexpected phone call from the future of dentistry
On a chilly March morning in Boston, 52-year-old Lauren Ortiz answered a call from her periodontist. Instead of scheduling another graft for the failing titanium implant in her lower molar, he invited her to enter an early-phase study in which a living replacement tooth would be coaxed to grow directly inside the empty socket. Ten years ago such a proposal would have sounded like science fiction; today it marks the leading edge of a quiet revolution that is bringing regenerative biology to the heart of everyday dentistry.
2. Why replace biology with
biology
?—Limitations of conventional implants
Modern dental implants, perfected by Per-Ingvar Brånemark’s discovery of osseointegration in the 1960s, rescued millions from edentulism. Yet they still behave like elegant prostheses, not organs:
- No proprioception. Implants fuse rigidly to bone and cannot relay pressure, texture, or temperature the way a periodontal-ligament-anchored tooth does. Chewing forces are therefore less finely tuned, and speech articulation can change.
- Surgical trauma and peri-implantitis. Drilling creates a thermal and mechanical insult; the titanium interface can harbor bacterial biofilm and provoke inflammatory bone loss over time.
- Static architecture. Unlike natural teeth, implants cannot remodel, erupt to compensate for wear, or repair micro-fractures. Bioengineered teeth aim to solve these shortcomings by re-creating the entire organ: enamel, dentin, pulp, vasculature, nerve fibers, cementum, and periodontal ligament, all patterned by the same developmental cues that build a deciduous or permanent tooth.
3. From dentures to developmental biology—A brief chronology
| Era | Milestone | Significance |
|---|---|---|
| 1700 BCE-19th C | Etruscan gold bands, ivory dentures, vulcanite plates | purely mechanical substitution |
| 1952 | Brånemark’s first osseointegrating titanium fixture | modern implantology foundation |
| 2002-2010 | Murine “tooth germs” generated from epithelial + mesenchymal cells (Takahashi/Ikeda, RIKEN) | proof-of-principle whole-tooth regeneration in mammals |
| 2013-2018 | Dental pulp stem-cell (DPSC) scaffolds regenerate dentin-pulp complex | partial tissue engineering |
| 2024 | Kyoto University launches first-in-human trial of USAG-1 antibody (TRG-035) to trigger third dentition | drug-based endogenous regrowth |
| 2025 | Tufts team publishes “smart implant” that reconnects to sensory nerve endings in rats | hybrid bio-prosthesis with proprioception |
4. Tooth development as a blueprint for engineering
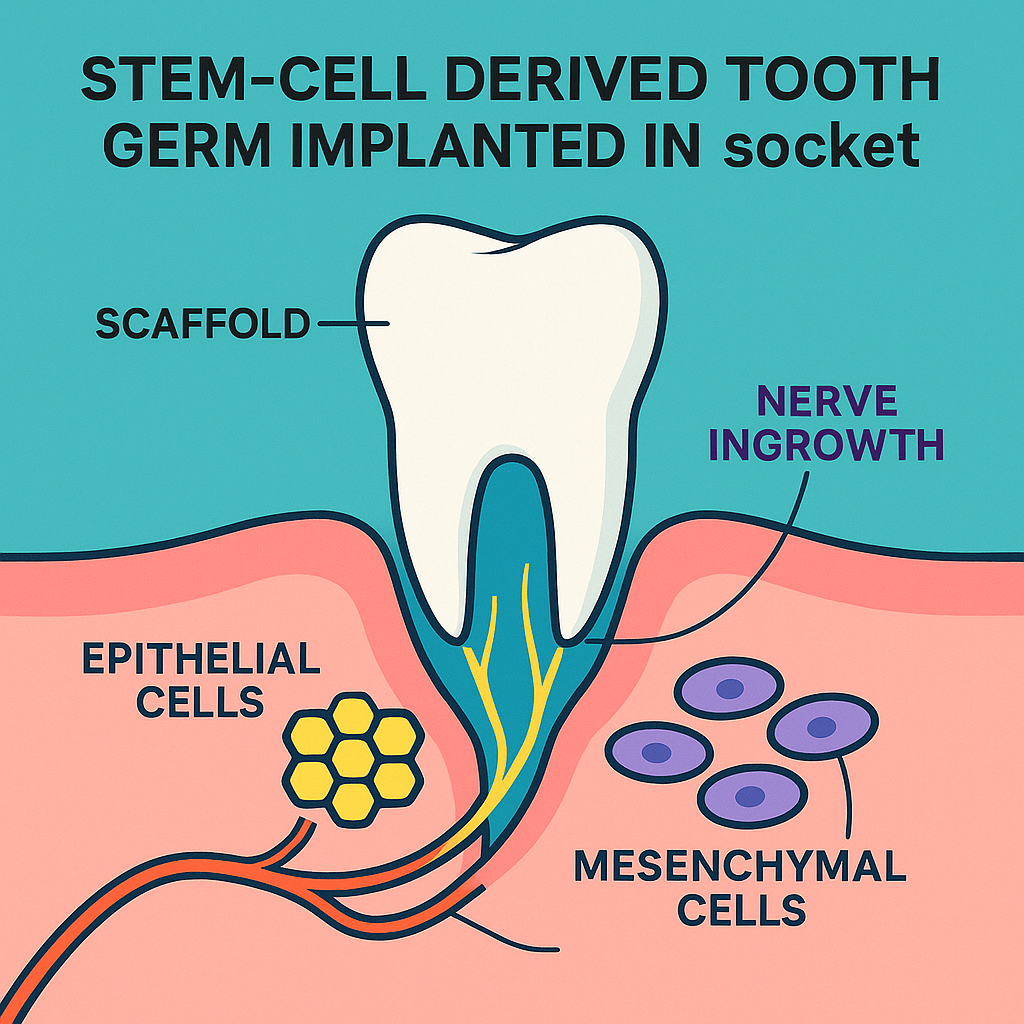
Natural odontogenesis is orchestrated by reciprocal signalling between oral epithelium and cranial neural-crest-derived mesenchyme. Key pathways—BMP4, FGF8, Wnt10a, Shh—drive morphogenesis from the dental lamina to a bell-stage tooth germ. Bioengineers replicate this choreography in three complementary strategies:
- Stem-cell–derived tooth germs
- Cell sources: autologous DPSCs, induced pluripotent stem cells (iPSCs), and embryonic stem cells.
- Assembly: co-culture epithelial and mesenchymal populations in a collagen or decellularized tooth matrix scaffold; 3-D bioprinting now enables precise cusp patterning.
- Preclinical data: erupted, enamel-covered teeth in mice and ferrets that connect to alveolar bone via a functional periodontal ligament and show nerve fiber ingrowth.
- Bioactive scaffolds and growth-factor gradients
- Poly-ε-caprolactone–hydroxyapatite frameworks loaded with BMP2 or VEGF encourage vascular invasion; electrospun nanofibers seeded with DPSCs accelerate pulp formation.
- Endogenous regrowth via molecular therapy
- USAG-1 monoclonal antibody (TRG-035): by blocking a protein that suppresses tooth-forming BMP/Wnt signals, dormant “third dentition” buds are re-awakened. In animals the drug generates perfectly shaped incisors; Phase I adult trial at Kyoto University (30 subjects missing ≥1 tooth) began in October 2024.
5. Achieving
integration
—vascular, neural, and immune hurdles
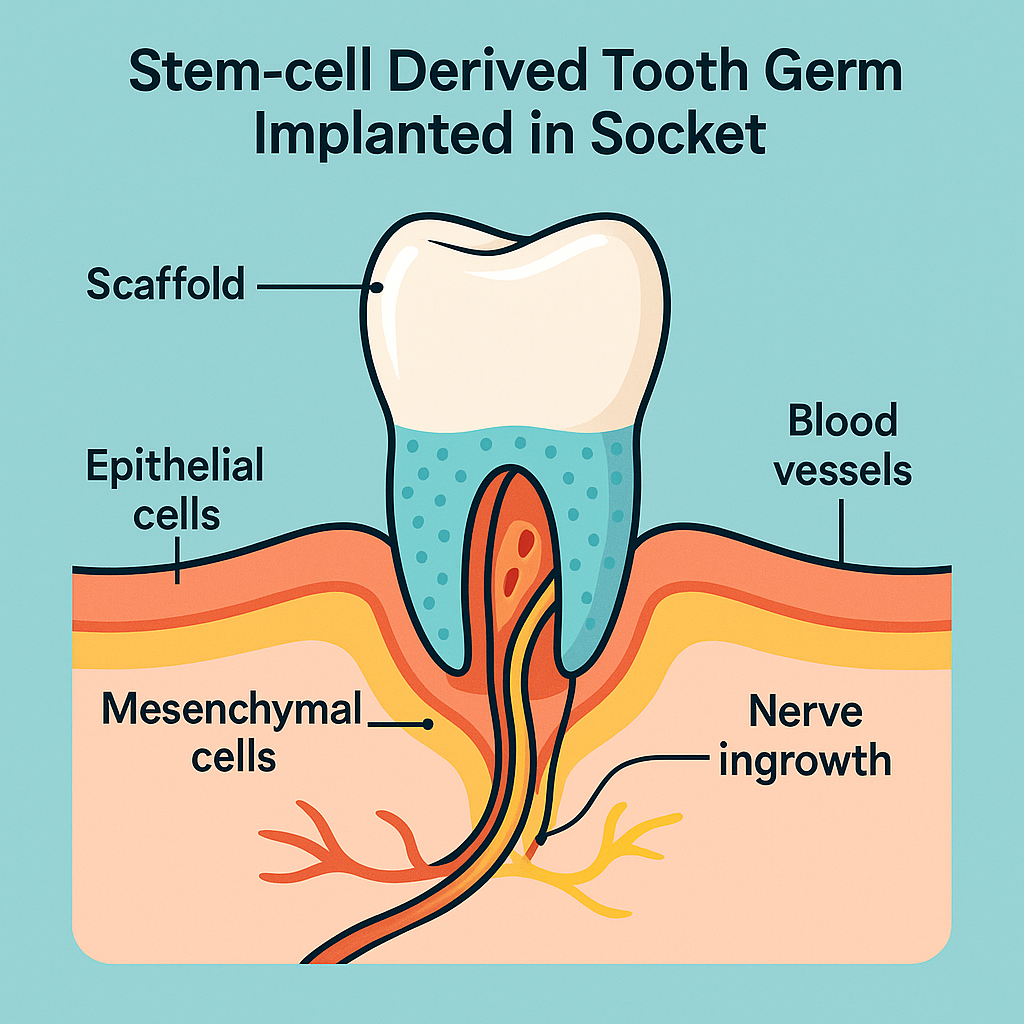
- Blood supply. Vascular endothelial growth factor (VEGF) microparticles embedded in the scaffold promote rapid inosculation with gingival capillaries, critical for pulp viability.
- Neural reconnection. The Tufts “smart implant” coats a titanium core with polyglycolic-acid nanofibers seeded with mesenchymal stem cells and neurotrophic factors, guiding trigeminal nerve sprouts into the newly formed periodontal ligament and pulp. Rodent models regained bite-force discrimination within eight weeks.
- Immune acceptance. Autologous iPSC-derived cells minimize rejection; decellularized porcine scaffolds risk xenoantigen exposure but are being glyco-engineered to reduce α-Gal epitopes.
- Eruption guidance. Controlled release of parathyroid-hormone-related protein (PTHrP) and tight occlusal splints direct vertical eruption to align with antagonistic teeth—essential to avoid malocclusion.
6. Clinical progress in 2025—where are we now?
| Platform | Status (mid-2025) | Key Data |
|---|---|---|
| Lab-grown tooth germ + scaffold (King’s College London) | Large-animal stage; human compassionate-use case scheduled 2026 | Successfully created in-vivo niche that supports full crown-root formation and periodontal ligament maturation. |
| USAG-1 antibody drug (Kyoto University) | Phase I, 30 adults | Safety and eruption kinetics primary endpoints; target commercial launch 2030 if Phase III succeeds. |
| Smart neuro-integrative implant (Tufts) | Preclinical; FDA Breakthrough Device designation pending | Restored 70 % of natural pressure sensitivity in rat models; “press-fit” insertion avoids bone drilling. |
| Hydrogel-based dentin-pulp regeneration (various) | Multiple early-phase trials for deep-caries lesions | Aimed at young permanent teeth to avert root-canal therapy. |
7. Benefits that extend beyond the tooth
- Functional proprioception means patients bite, chew, and enunciate with the same sensory feedback they enjoyed before tooth loss.
- Adaptive remodeling allows the regenerated tooth to compensate for wear and maintain occlusion over decades—an ability implants lack.
- Reduced peri-implant disease because the periodontal ligament contains its own immune surveillance and shock-absorbing fibers.
- Psychological comfort. Patients consistently report that a living replacement “feels like me,” reducing foreign-body awareness and improving self-confidence.
8. Remaining scientific and regulatory challenges
- Scaling and cost. A single autologous tooth-germ build presently costs ~US $8 000 in lab consumables. High-throughput bioreactors and allogeneic iPSC banks may drive costs down.
- Long-term safety. iPSC derivatives carry a theoretical teratoma risk; rigorous purification and suicide-gene safeguards are under review by regulators.
- Occlusion and orthodontics. Controlling eruption trajectory is harder in multicusp premolars and molars than in incisors; adjunct clear-aligner therapy may become routine.
- Reimbursement pathway. Because bioengineered teeth blur the line between biologic drug and medical device, the FDA and EMA are drafting hybrid regulatory frameworks similar to those used for autologous CART-cell therapies.
9. Ethical perspectives—regeneration vs. enhancement
If we can regrow enamel harder than nature’s, or tweak dentin to release antimicrobial peptides, should we? Dental ethicists argue that first-generation therapies must focus on restoring baseline human dentition. Enhancement (super-teeth, luminescent enamel, embedded biosensors) raises equity and consent issues that mirror debates in gene editing.
10. The road to your dentist’s chair
2025-2027 – Safety read-outs from Kyoto’s TRG-035 and release of first‐in‐human tooth-germ scaffold data.
2028-2030 – Pivotal trials comparing bioengineered molars with titanium implants on masticatory efficiency, QoL, and cost-effectiveness.
Early 2030s – Insurance codes and postgraduate curricula adapt; regenerative dentistry becomes a recognized specialty.
11. Conclusion
Dentistry is shifting from carpentry to developmental biology. By harnessing stem-cell engineering, smart biomaterials, and molecular reactivation of dormant buds, researchers are on the verge of making living tooth replacement routine. For patients like Lauren Ortiz, the promise is profound: not merely an elegant prosthesis, but the genuine article—a new tooth that grows, feels, and functions as though it had always been there.
Prepared June 12 2025. Sources include peer-reviewed reports and press releases from Tufts University School of Dental Medicine, King’s College London, Kyoto University Hospital, and recent scientific reviews on whole-tooth regeneration.